Rare-earth elements Ln (Ln: Sc, Y, and lanthanides) play crucial roles in a variety of today’s high-tech applications, such as magnetism, luminescence and catalysis. Rare-earth organometallic chemistry attracts intensive interests, which not only expands our chemical knowledge on rare-earth elements but also provides new rare-earth metal complexes for material science or rare-earth catalysts in organic and polymer synthesis. Currently, our group’s research mainly focuses on two projects listed as below:
Project A: Synthesis, bonding properties and reactivity of rare-earth metal-ligand multiple bonds
The synthesis and properties of transition metal-ligand multiple bonds (M=E/M≡E; M = transition metal, E = main group element) is one of the most vibrant areas of modern organometallic chemistry. A great number of transition metal-ligand multiple-bonding species have been synthesized but not involving rare-earth metals. The synthesis of rare-earth metal ones lagged behind transition-metal and actinide congeners for decades. The scarcity is mainly attributed to energy mismatching between the frontier orbitals of the rare-earth metal and the ligand atoms. This renders the putative Ln=E/Ln≡E bonds extremely reactive, thus resulting in the formation of aggregates and/or reacting with ligand/environment, quenching the multiple bond character. But, once such complexes can be synthesized, they exhibit unique structural properties as well as high reactivity. We are interesting in the synthesis, bonding properties and reactivity of rare-earth metal complexes bearing multiply-bonded main-group ligands as show below. We have had some interesting achievements in the imido and alkylidene complexes, see our representative publications (Chem. Commun. 2010, 46, 4469; Angew. Chem. Int. Ed. 2011, 50, 7677; J. Am. Chem. Soc. 2014, 136, 10894; J. Am. Chem. Soc. 2017, 139, 1081; J. Am. Chem. Soc. 2017, 139, 17759; Acc. Chem. Res. 2018, 51, 557).
Project B: Rare-earth metal catalyzed organic reactions
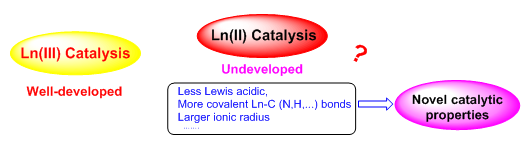
 (1) Divalent rare-earth metal complexes catalyzed organic reactions: Rare-earth metal complexes have been employed as the catalysts for many organic and polymer synthesis. These complexes are mainly trivalent. For the divalent rare-earth metal complexes, they are well known as the reductants in stoichiometric reactions, but their applications in catalysis are very limited. The change in the metal oxidation state will certainly change the catalytic properties of the rare-earth metal complexes and may bring up some reactivity beyond that found for the trivalent ones. We are recently interested in this field, and found that a newly synthesized divalent ytterbium alkyl complex exhibits unprecedentedly high catalytic activity towards the homo- and cross-coupling of primary arylsilanes (J. Am. Chem. Soc. 2019, 141, 138).
(1) Divalent rare-earth metal complexes catalyzed organic reactions: Rare-earth metal complexes have been employed as the catalysts for many organic and polymer synthesis. These complexes are mainly trivalent. For the divalent rare-earth metal complexes, they are well known as the reductants in stoichiometric reactions, but their applications in catalysis are very limited. The change in the metal oxidation state will certainly change the catalytic properties of the rare-earth metal complexes and may bring up some reactivity beyond that found for the trivalent ones. We are recently interested in this field, and found that a newly synthesized divalent ytterbium alkyl complex exhibits unprecedentedly high catalytic activity towards the homo- and cross-coupling of primary arylsilanes (J. Am. Chem. Soc. 2019, 141, 138).
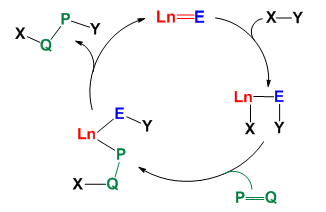
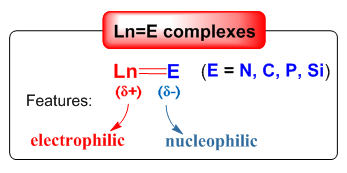
 (2) Catalysis based on the Ln=E bonding: The developed rare-earth metal catalyzed organic reactions are generally based on the Ln-E -bond metathesis or rare-earth metal complexes being the Lewis acid catalysts. The recent emergence of Ln=E complexes provides an opportunity to design the new catalytic cycle for rare-earth metal catalyzed reactions as shown below. The highly polarized interaction in the Ln=E bonds enable the complexes to activate inert bonds, which has been recently demonstrated in stoichiometric reactions (Acc. Chem. Res. 2018, 51, 557). The second step in the catalytic reaction, the addition of the Ln-X single bond to the unsaturated bond, is well known.
(2) Catalysis based on the Ln=E bonding: The developed rare-earth metal catalyzed organic reactions are generally based on the Ln-E -bond metathesis or rare-earth metal complexes being the Lewis acid catalysts. The recent emergence of Ln=E complexes provides an opportunity to design the new catalytic cycle for rare-earth metal catalyzed reactions as shown below. The highly polarized interaction in the Ln=E bonds enable the complexes to activate inert bonds, which has been recently demonstrated in stoichiometric reactions (Acc. Chem. Res. 2018, 51, 557). The second step in the catalytic reaction, the addition of the Ln-X single bond to the unsaturated bond, is well known.